HDV an infection induces probably the most severe kind of human viral hepatitis. However, the precise causes for the severity of the illness stay unknown. Recently, we developed an HDV replication mouse model in which, for the primary time, liver damage was detected.
HDV and HBV replication-competent genomes and HDV antigens had been delivered to mouse hepatocytes utilizing adeno-associated vectors (AAVs). Aminotransferase elevation, liver histopathology, and hepatocyte dying had been evaluated and the immune infiltrate was characterised. Liver transcriptomic evaluation was carried out.
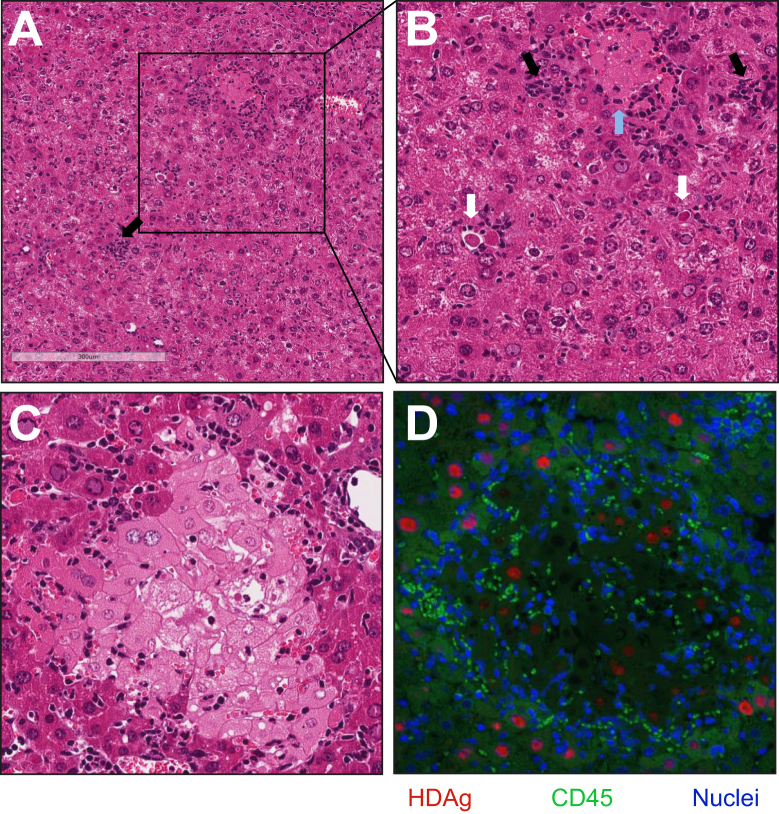
Mice poor for various mobile and molecular parts of the immune system, in addition to depletion and inhibition research, had been employed to elucidate the causes of HDV-mediated liver damage.AAV-mediated HBV/HDV coinfection brought on hepatocyte necrosis and apoptosis.
Activated T lymphocytes, pure killer cells, and proinflammatory macrophages accounted for almost all of the inflammatory infiltrate. However, depletion research and the use of completely different knockout mice indicated that neither T cells, pure killer cells nor macrophages had been obligatory for HDV-induced liver damage.
Transcriptomic evaluation revealed a robust activation of sort I and II interferon (IFN) and tumor necrosis issue (TNF)-α pathways in HBV/HDV-coinfected mice.
While the absence of IFN signaling had no impact, the use of a TNF-α antagonist resulted in a important discount of HDV-associated liver damage. Furthermore, hepatic expression of HDAg resulted in the induction of severe liver damage, which was T cell- and TNF-α-independent.
Both host (TNF-α) and viral (HDV antigens) elements play a related function in HDV-induced liver damage. Importantly, pharmacological inhibition of TNF-α could supply a pretty technique to assist management of HDV-induced acute liver damage.
Chronic hepatitis delta constitutes probably the most severe kind of viral hepatitis. There is restricted information on the mechanism concerned in hepatitis delta virus (HDV)-induced liver pathology. Our information point out that a cytokine (TNF-α) and HDV antigens play a related function in HDV-induced liver damage.
Optimizing the SERS Performance of 3D Substrates by way of Tunable 3D Plasmonic Coupling towards Label-free Liver Cancer Cell Classification.
Three-dimensional (3D) plasmonic nanostructures are rising as wonderful surface-enhanced Raman spectroscopy (SERS) substrates for chemical and biomedical purposes. However, the correlation of the 3D (together with each of the in-plane and out-of-plane) plasmonic coupling with the SERS properties to deepen the understanding of 3D SERS substrates stays a problem.
Here, we carry out correlated research of 3D plasmonic coupling and SERS properties of the 3D hierarchical SERS substrates by tuning the multiscale structural parts. The impact of 0D (the scale of constructing blocks), 1D (the thickness of the 3D substrates) and 2D (the composition of particular person monolayers) structural parts on 3D plasmonic coupling are studied by measuring the UV-Vis-NIR spectroscopy and SERS efficiency.
It exhibits that each of the extinction spectra and SERS enhancement are tuned on the 3D structural degree.
It is demonstrated that the plasmonic resonance wavelength (PRW) stemmed from the 3D plasmonic coupling is correlated with the SERS averaged floor enhancement issue (ASEF), and which is improved by over 10-fold on the optimum 3D nanostructure.
The optimized substrate is used to quantitatively analyze two small organic molecules. Moreover, as a proof-of-concept research, the substrate is first utilized to distinguish between dwelling liver regular and most cancers cells with a excessive prediction accuracy by way of the spectral options of the cell membranes and the metabolites secreted exterior the cells.
We count on that the tuning of plasmonic coupling at 3D degree can open up new routes to design excessive efficiency SERS substrates for large purposes.
